
Explain the Gas Law
The gas laws are a set of physical laws that describe the behavior of ideal gases. They are a simplification of the kinetic theory of gases, which describes the behavior of gases based on the motion of their individual molecules. The
s laws are used in many different fields, including chemistry, physics, and engineering.
1: There are four main gas laws
2: Boyle's law
Boyle's law states that the pressure of a fixed mass of gas held at a constant temperature is inversely proportional to its volume. In other words, as the volume of a gas decreases, its pressure increases, and vice versa.
3: Charles' law
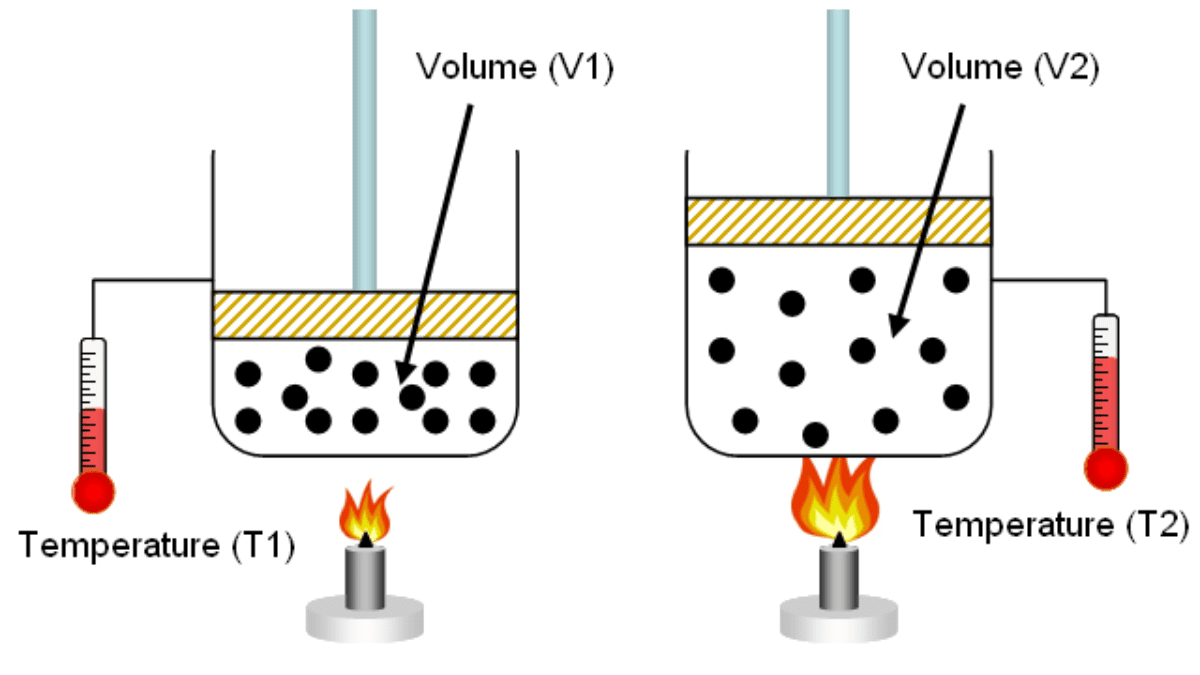
Charles' law states that the volume of a fixed mass of gas held at a constant pressure is directly proportional to its temperature. In other words, as the temperature of a gas increases, its volume increases, and vice versa.
4: Gay-Lussac's law
Gay-Lussac's law states that the pressure of a fixed mass of gas held at a constant volume is directly proportional to its temperature. In other words, as the temperature of a gas increases, its pressure increases, and vice versa.
Avogadro's law: Avogadro's law states that equal volumes of gases held at the same temperature and pressure contain the same number of molecules.
5: These laws can be combined into a single equation called the ideal gas equation.
PV = nRT
where:
1: P is the pressure of the gas
2: V is the volume of the gas
3: n is the number of moles of gas
4: R is the ideal gas constant
5: T is the temperature of the gas
The ideal gas equation is a very important equation in chemistry and physics, and it is used in a wide variety of applications. For example, it can be used to calculate the pressure of a gas in a container, the volume of a gas at a given temperature and pressure, or the number of moles of gas in a sample of gas.
6: Here are some examples of how the gas laws can be used in the real world.
7: A weather forecaster might use the gas laws to predict how the temperature and pressure of the air will change over time.
8: A chemist might use the gas laws to calculate the amount of gas that will be produced in a chemical reaction.
9: An engineer might use the gas laws to design a system for storing and transporting natural gas.
The gas laws are a powerful tool that can be used to understand and predict the behavior of gases. They are an essential part of chemistry and physics, and they have many applications in the real world.