
Coulomb's Law
Coulomb's law, a cornerstone of electromagnetism, describes the force between two point charges. This fundamental principle, formulated by French physicist Charles-Augustin de Coulomb in the late 18th century, has revolutionized our understanding of electricity and its implications across various scientific fields.
Coulomb's Law
The Essence of Coulomb's Law
Coulomb's law states that the magnitude of the electrostatic force (F) between two point charges, q1 and q2, is directly proportional to the product of the magnitudes of the charges (q1 * q2) and inversely proportional to the square of the distance (r) between them. Mathematically, this can be expressed as:
F = k * (q1 * q2) / r^2
where k is Coulomb's constant, approximately 8.98755 × 10^9 N·m^2/C^2, a fundamental constant of nature.
The force is attractive if the charges have opposite signs and repulsive if the charges have the same sign. This is consistent with the observation that oppositely charged objects attract each other, while objects with the same charge repel each other.
Delving into the Implications of Coulomb's Law
Coulomb's law has profound implications in various fields of science and technology:
Electricity and Electronics: Coulomb's law provides the foundation for understanding the behavior of electric charges in circuits, transistors, and other electronic components, enabling the development of modern electronics.
Atomic Structure: Coulomb's law plays a crucial role in explaining the electrostatic forces that bind electrons to the nucleus of an atom, forming the building blocks of matter.
Chemical Bonding: Coulomb's law governs the electrostatic interactions between atoms that lead to the formation of chemical bonds, shaping the properties and interactions of molecules.
Electromagnetism: Coulomb's law forms the basis of electromagnetism, describing the relationship between electric charges and electric fields, which are essential for understanding electromagnetic phenomena, including light, radio waves, and other electromagnetic radiation.
Material Science: Coulomb's law is crucial in understanding the electrical properties of materials, enabling the development of new materials with tailored electrical properties for various applications.
Conclusion: Coulomb's Law – A Guiding Principle in Electromagnetism
Coulomb's law, with its simplicity and elegance, has revolutionized our understanding of electromagnetism and its far-reaching implications across science and technology. From the behavior of electrons in atoms to the functioning of electronic devices, Coulomb's law has provided a guiding principle in unraveling the mysteries of electric charges and their interactions. As we continue to explore the depths of electromagnetism, Coulomb's law will undoubtedly remain a cornerstone of our understanding and advancement in this fascinating field.
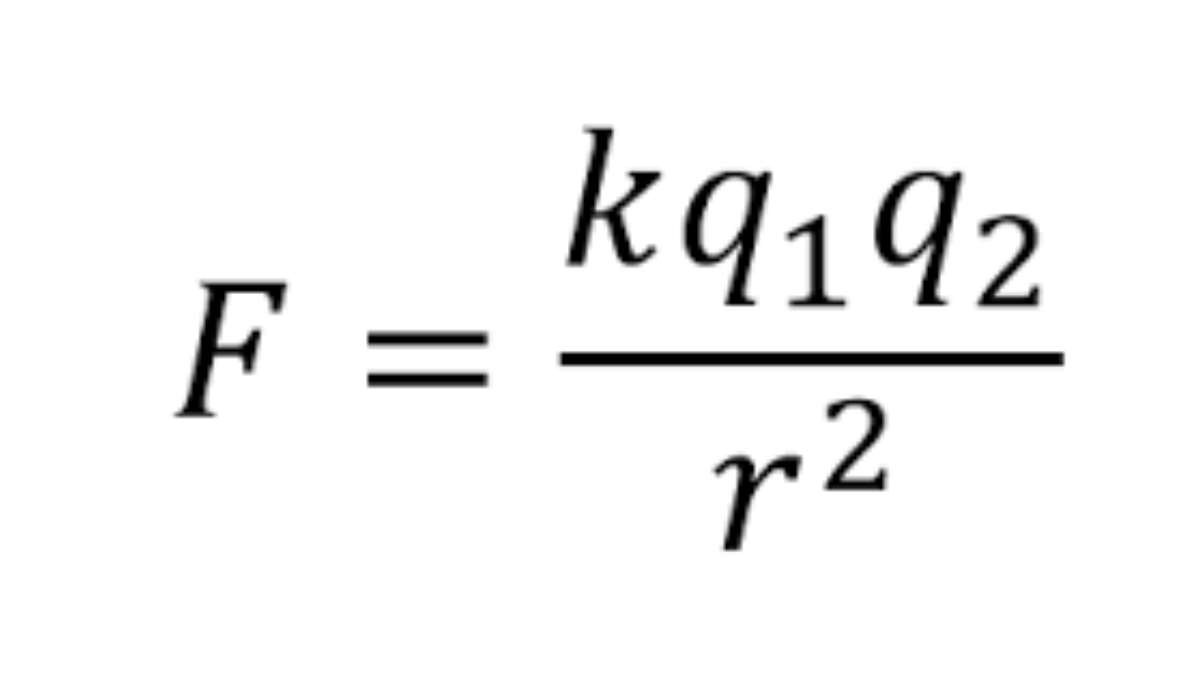
Appreciate the creator