Boly's Law
Introduction:
In the world of physics, certain laws govern the behavior of gases, and one such fundamental principle is Boyle's Law. Named after the Irish scientist Robert Boyle, this law provides insights into how the pressure and volume of a gas are interconnected when temperature remains constant. For 10th-grade students studying physics, grasping the basics of Boyle's Law is essential as it lays the groundwork for understanding various real-world applications.
Boyle's Law Equation:
The Equation: PV=k
What it Means:
P is pressure,
V is volume,
k is a constant.
Understanding the Equation:
Pressure (P): This is the force exerted per unit area by the gas molecules colliding with the walls of the container. If you increase the pressure on a gas, like squeezing a balloon, the volume it occupies will decrease.
Volume (V): This represents the amount of space the gas occupies. As per Boyle's Law, if you change the volume of a gas, the pressure it exerts will respond inversely. If you allow a gas to expand into a larger space, the pressure decreases.
Constant (k): The product of pressure and volume is always constant as long as the temperature remains constant. It's like a magic number that stays the same no matter how much you change the pressure or volume. Mathematically, k is the initial product of pressure and volume before any changes occur.
The Inverse Relationship:
Boyle's Law describes an inverse relationship between pressure and volume when temperature is held constant. In simpler terms, as one of these variables increases, the other decreases, and vice versa.
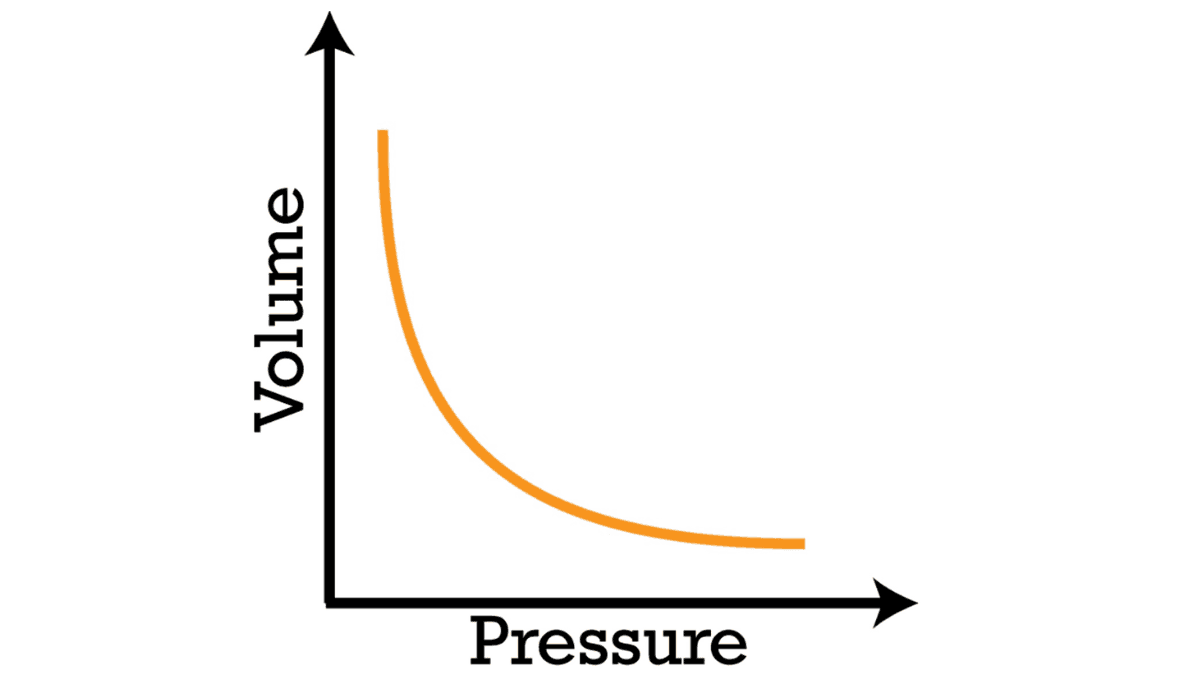
Graphical Representation:
If you were to graph the relationship between pressure and volume, you would get a hyperbola. The curve shows how changes in pressure correspond to changes in volume and vice versa. As one variable increases, the other decreases to maintain the product PV constant.
Real-World Examples:
Balloon Experiment: Squeeze a balloon gently, and you'll notice it gets smaller. Release the squeeze, and it expands. This is Boyle's Law in action.
Scuba Diving: As you descend underwater, the pressure increases, and according to Boyle's Law, the volume of air in your scuba tank decreases.
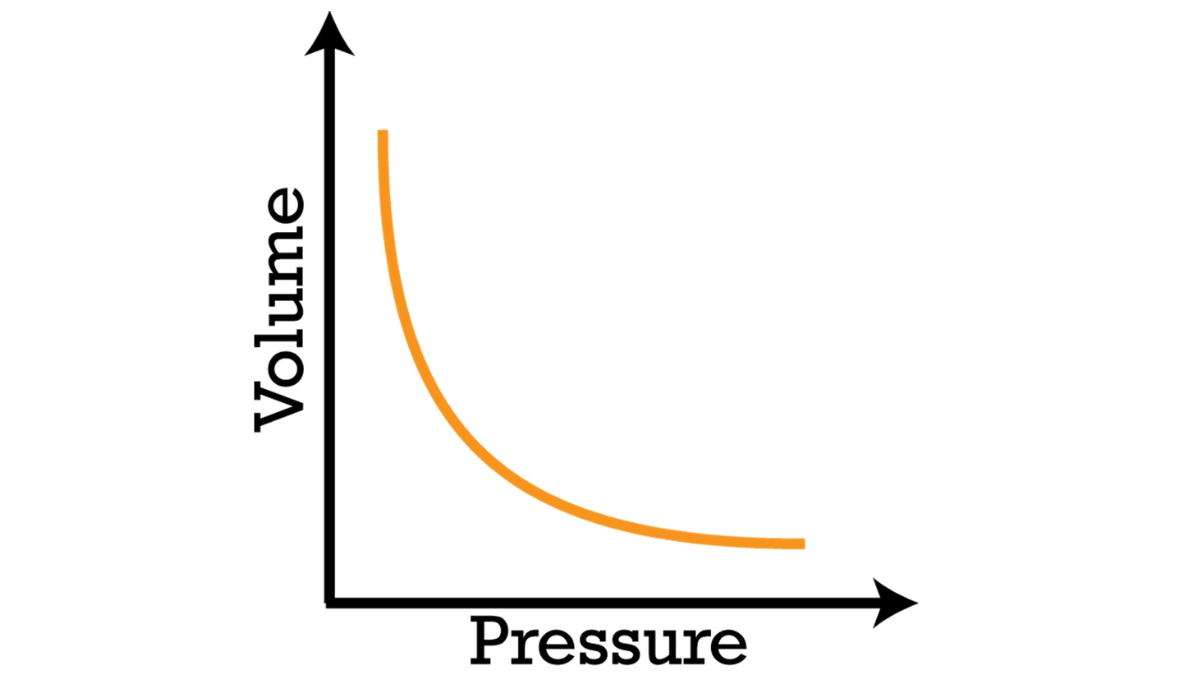
shows boyle' law
Appreciate the creator